Buy These 2 Stocks Before They Jump 40%, Says Goldman Sachs
[ad_1]
Markets turned down in the first six months of this year, but they’ve trended up in July. Despite Friday’s pullback, the monthly gains are solid, almost 5% on the S&P 500 and 7.5% on the NASDAQ, marking a turnaround from the long drop we saw earlier.
The question investors have is, is this turnaround real, or just a temporary gain in a larger bearish trend. That remains to be seen, but either way, even if the market reverts to its bearish trend, there will be opportunities for investors – finding them will be the key to success.
There’s no way to truly predict how a stock will perform. The old adage says, ‘Past success is no guarantee of future gains.’ But that works both ways, and recent losses don’t necessarily predict further declines. So perhaps we should turn to the experts, and find out what some of Wall Street’s stock pros are picking as winners right now.
The analysts at banking giant Goldman Sachs have been looking for stocks with the potential for hot gains in the coming months – on the order of 40% or more. The GS experts have taken an upbeat look on individual stocks, despite this market’s full-year downward trend. Now let’s get a feel for their optimism by using the TipRanks database to pull up the latest details on two of their picks. Here they are, alongside the analysts’ commentary.
Allogene Therapeutics, Inc. (ALLO)
The first stock we’ll look at, Allogene Therapeutics, is a biopharmaceutical company pursuing research in cancer immunotherapies, using allogenic chimeric antigen receptor T-cells, or AlloCAR T, to develop new agents for disease treatment. These are purpose-designed precision medicines that work with the patient’s own immune system to attack cancers. The company is at the clinical stage, with several drug candidates undergoing human clinical trials.
In recent news on the clinical front, Allogene’s most advanced candidate, ALLO-501A, received regenerative medicine advanced therapy (RMAT) designation from the FDA, giving the program an expedited status. ALLO-501A’s new designation followed on a positive data release from the ALPHA2 trial, which is testing the drug on patients with relapsed or refractory Large B cell Lymphoma (LBCL). The data showed that AlloCAR T therapies are safe and effective, and produce durable patient responses. The company plans to initiate a Phase 2 pivotal trial this year.
In another recent clinical program update, Allogene announced that its new drug candidate ALLO-316 has started the Phase 1 TRAVERSE trial, to evaluate safety, tolerability, anti-tumor efficacy, pharmacokinetics, and pharmacodynamics. This drug candidate is the company’s first to target solid tumors, and the TRAVERSE trial has enrolled patients with advanced or metastatic clear cell renal cell carcinoma (RCC). The trial is now studying its second cohort, and further enrollment is ongoing. ALLO-316 was granted the FDA’s Fast Track designation in March of this year.
Finally, the company is studying ALLO-715 in the treatment of multiple myeloma. This drug candidate is the subject of the UNIVERSAL trial. ALLO-715 has promise in targeting BCMA (B cell maturation antigen) for patient treatment.
Allogene’s extensive clinical program is expensive, and the company spent some $60 million on R&D in 1Q22. This was paired with an additional $19.9 million in G&A spending. While high, this spending is supported by the company’s cash and liquid asset holdings, which totaled $733.1 million at the end of the quarter, suggesting a cash runway for operations extending through the next 9 quarters.
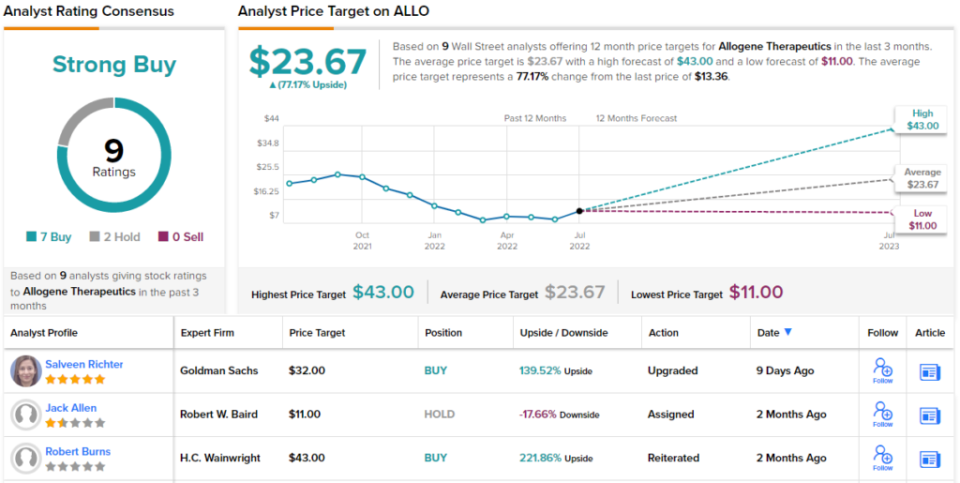
In her coverage for Goldman, 5-star analyst Salveen Richter points out the key upcoming catalysts here, writing, “…we anticipate a period of execution on lead allogeneic CAR T programs ALLO-501A (anti-CD-19) … where FDA alignment on and initiation of the pivotal trials in mid-22 pave a path toward approval, and ALLO-715 (anti-BCMA) … where clarity on the forward path is expected by YE22 per longer-term follow-up monotherapy data… Separately, we note first data from the Ph1 ALLO-316 TRAVERSE study in renal cell carcinoma in 2023 could unlock the solid tumor vertical.”
Richter doesn’t stop with positive comments. She upgrades her stance on ALLO from Neutral to Buy and has a $32 price target that implies a 139% upside for the next 12 months. (To watch Richter’s track record, click here.)
This immunotherapy researcher has picked up 9 recent analyst reviews, and these break down to 7 Buys against just 2 Holds for a Strong Buy consensus rating. The shares have an average price target of $23.67 and a current trading price of $13.36, indicating a 77% upside potential on the one-year time frame. (See Allogene’s stock forecast at TipRanks.)
Prometheus Biosciences (RXDX)
The next Goldman pick we’re looking at is another biopharma in the precision medicine niche. Prometheus is working on new treatments for immune-related gastrointestinal conditions, with a focus on inflammatory bowel diseases (IBD). Most of Prometheus’ program is in early, preclinical stages, but the company does have one drug candidate, PRA023, currently undergoing three human clinical trials to treat Ulcerative Colitis, Crohn’s Disease, and Systemic Sclerosis-associated Interstitial Lung Disease (SSc-ILD). The company uses a biomarker-targeted therapeutic approach, based upon a patient’s biomarker profile. This patient-centric mode offers the promise of transformed patient outcomes.
Prometheus has recently reported several updates to its clinical trial programs of PRA023. First, the company has initiated a third Phase 2 study of the drug candidate, focusing on SSc-ILD. The study was initiated in March of this year, and top line results are expected in 1H24.
Next was the announcement that the FDA had granted Fast Track designation to PRA023 on the SSc-ILD track.
Finally, the company received a US patent for PRA023. The new patent covers “claims directed to methods of treating Crohn’s disease or ulcerative colitis by administering inhibitors of tumor necrosis factor-like cytokine 1A (TL1A) to patients selected by a defined companion diagnostic test.” Intellectual property is a vital asset in biopharmacology, and this patent helps protect Prometheus’ investment in PRA023 until 2040.
Chris Shibutani, another of Goldman’s 5-star biotech experts, writes of Prometheus, “We have a favorable view of RXDX’s to bring precision medicine to UC and CD, given the markets are large and well-developed, and the current standard of care therapies elicit modest rates of clinical remission. We believe there is significant value from PRA023 in IBD indications, and see upside potential expanding into SSc-ILD, as well as the company’s ability to leverage their platform to develop novel agents and diagnostics to address additional patient populations.”
With these upbeat comments in mind, it’s no surprise that Shibutani initiates his firm’s coverage of RXDX with a Buy rating, while his $51 price target suggests a one-year upside potential of 47%. (To watch Shibutani’s track record, click here.)
It’s clear from the unanimous Strong Buy consensus rating on this stock that Wall Street is in broad agreement with the bullish Goldman view; all 7 of the recent analyst reviews here are positive. The stock is currently trading for $34.65, and its $52 average price target implies a 12-month gain of 50% lies ahead for Prometheus. (See Prometheus’ stock forecast at TipRanks.)

To find good ideas for stocks trading at attractive valuations, visit TipRanks’ Best Stocks to Buy, a newly launched tool that unites all of TipRanks’ equity insights.
Disclaimer: The opinions expressed in this article are solely those of the featured analysts. The content is intended to be used for informational purposes only. It is very important to do your own analysis before making any investment.
Source link